|
|
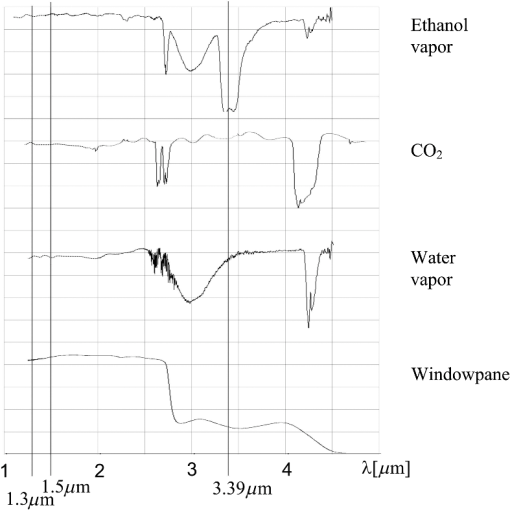
1.IntroductionThere are many papers dealing with stand-off detection of different chemical and biological compounds with the use of a monochromatic laser beam at a wavelength fitting into the absorption spectra of these materials.1–6 At the same time, many new developments in lasers that can be used in this application have been achieved in recent years.7–12 Thus many devices based on this technology were presented in laboratories as well as applied to civil industry, environmental protection, and military. The differential absorption lidars (DIAL) are the most often used systems to monitor the atmosphere and detect contaminants in the form of gas, aerosol, fume, or dust. They make use of the well-known physical phenomenon of “difference absorption” using two laser beams at different wavelengths. Among many advantages of these systems are the ability to analyze the sample without the necessity to collect it, real-time investigation as well as integration of electro-optical systems and full automation of the measurement. Moreover the time needed to take measurements is equivalent to the speed of light; thus such lidars can be successfully used to face alcohol abuse among drivers. Ethyl alcohol is a chemical compound that is characterized by high volatility, reflection of which is its high concentration on exhalation of people who have consumed it. Thus the car cabin where the drunken people are located is expected to be filled with air having some concentration of alcohol. In such situations, the simple DIAL system seems to work properly enough to detect the concentration of alcohol in the cabin, but it is not so. In order to assure the reliable results of measurements, the physical phenomena appearing in the side window panes of the car have to be taken into account. In this connection, a new method of detection was proposed and described in this article as well as in three patents and a study.13–15 2.Spectral IssuesTo effectively detect chemicals in cars using the difference absorption method, it is essential to know not only its transmission spectra but also the transmission spectra of accompanying substances as well as transmission spectra of the side window panes of cars. In this case the accompanying substances that have to be taken into consideration are carbon dioxide () and water vapor (). In Fig. 1, the measured transmission spectra of vapor of ethanol (rectified spirit), , vapor of , and window pane are presented. To measure the spectra, the first three chemical compounds were placed inside a 12-cm-long glass pipe terminated by quartz windows. The windowpane used for experiments was made of flot glass by Pilkington company and is the most often used window pane in cars. Ethyl alcohol is characterized by a quite wide absorption band around the 3.39 μm wavelength, which is mainly caused by stretching vibrations of OH, CH, and CO bonds being included in molecule.16 This situation is very useful for the choice of the wavelength absorbed by the alcohol, but at the same time it implies the necessity of choosing the reference wavelength, which lies relatively far from the previous one. The absorption band around 3 μm wavelength is caused by water that occurs in rectified spirit. Keeping in mind the measured transmission spectra and availability of lasers, it seems that the He–Ne laser generating at 3.39 μm wavelength, strongly absorbed by the alcohol, is the best option. For reference beam, a laser diode generation at 1.5 μm wavelength was chosen. Using only two wavelengths is not enough to detect alcohol because of the window pane, which has different transmission for these wavelengths as well as different thicknesses in different cars. If the thickness was the same for all cars or the transmission was the same for both wavelengths, there would not be any problem with the detection. However, the differences are quite big and using the third wavelength, which will enable measurement of the thickness of the windowpane, becomes necessary. The third wavelength should have a transmission different from that of 1.5 μm wavelength and lie outside the absorption bands of alcohol, , and . Laser diode generation at 1.3 μm wavelength seems to be a reasonable solution. The chosen wavelengths are indicated in Fig. 1 by straight vertical lines. Very important phenomena that should be taken into account are dusting and misting over window panes. To face these problems, transmission spectra of clean window panes and those covered with water vapor, road dust, and graphite dust were measured and presented in Fig. 2. Fig. 2Transmission spectra of clean windowpane (1), windowpane covered with water vapor (2), windowpane covered with road dust (3), windowpane covered with graphite dust (4). 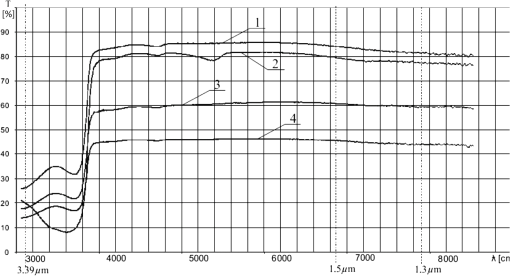 To calculate the transmission of the layers of dust and water vapor, the transmissions of the covered window panes were divided by the transmission of the clean window pane at selected wavelengths. The results of the calculations are presented in Table 1, where is the transmission of the clean window pane, is the transmission of the window pane covered with water vapor, is the transmission of the window pane covered with road dust, is the transmission of the window pane covered with graphite dust, is the transmission of water vapor, is the transmission of road dust, is the transmission of graphite dust, and is the wavelength. Table 1Transmissions of the layers of dust and water vapor.
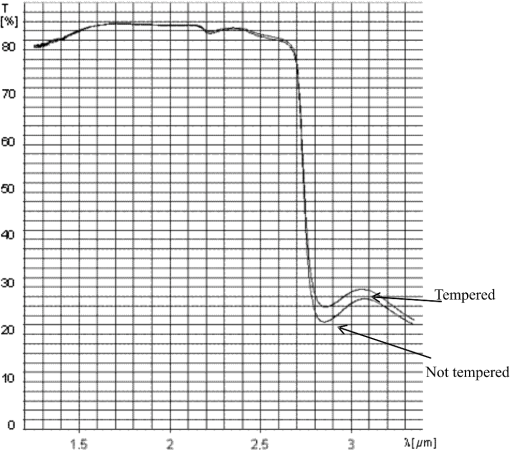
Looking at the results of calculations it seems that the appearance of dust does not have any influence on the results of measurement of the concentration of alcohol, because its transmission at the investigated wavelengths is almost the same. Water vapor has lower transmission at 3.39 μm and then at 1.3 and 1.5 μm wavelengths, which is not desired; however, the difference is so small that it should not interfere with the detection of alcohol. 3.Optical Parameters of WindowpaneThe window pane used for the experiments was the same as in the previous investigations. The transmission spectra of the window pane before and after thermal processing (tempering and bending) with thickness of 3.15 mm were measured and are presented in Fig. 3. In Table 2 the transmissions of the window panes at the investigated wavelengths are presented. As can be seen from the table, the tempering does not significantly change the transmission of the window pane at any of the investigated wavelengths. Thus for the next experiments, a window pane that was not tempered was used since it is easier to machine (cutting, grinding, polishing). Table 2Transmissions of windowpanes at the investigated wavelengths.
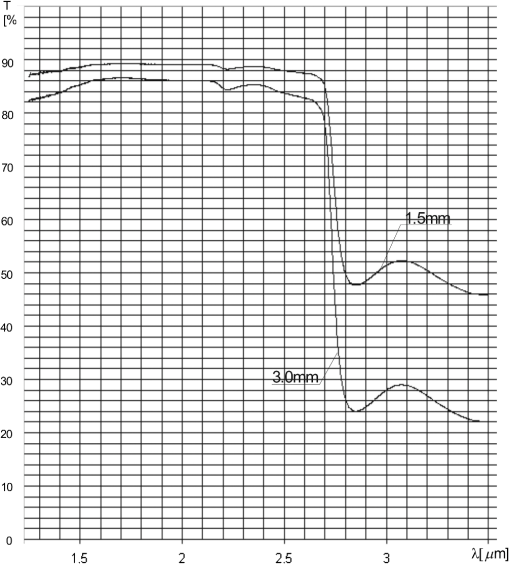
Because the optical parameters of the flot glass used for the production of the window panes are not available from the producer in the investigated spectral range, they had to be measured. The parameters that are indispensable for accurate detection of alcohol are absorption coefficient and refractive index. In order to calculate the absorption coefficient of the window pane, the transmission spectra of two samples of flot glass with thickness of and were measured. The transmission spectra are presented in Fig. 4, while in Table 3 the transmissions at the investigated wavelengths are shown. Table 3Transmissions T(λ) at the investigated wavelengths λ.
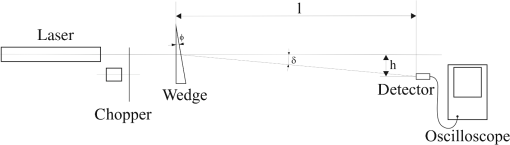
Assuming that there is no scattering on the surface of the sample and inside it, the intensity of the radiation going through it can be written as where is the intensity of the incident radiation, is the reflection coefficient, is the absorption coefficient, and is the thickness of the sample.In the investigated situation, and the transmission of the sample can be described asIntroducing the following notation for radiation at a specified wavelength , Eq. (4) can be written as whereFor the samples with thickness of and , After dividing Eq. (7) by Eq. (8), ThusTaking into account the transmissions from Table 3, the absorption coefficients for the investigated wavelengths are Squaring both sides of Eq. (8), Thus for three investigated wavelengths, The refractive index of the window pane was determined by the use of the experimental setup shown in Fig. 5. During the experiment the laser beams were passing through the wedge made of flot glass with the vertex angle of and the angular deviation was measured by a detector. The experiment was repeated for three investigated wavelengths. The measured angular deviations were equal to Using the expression the refractive indexes can be calculated to be equal toThe calculated absorption coefficients, refractive indexes, and transmissions are presented in Table 4. Table 4Calculated absorption coefficients, refractive indexes, and transmissions T0(λ).
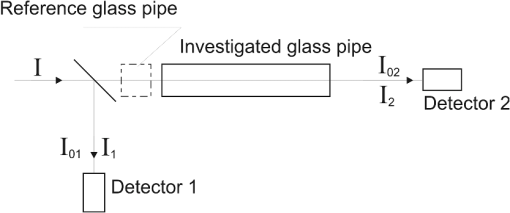
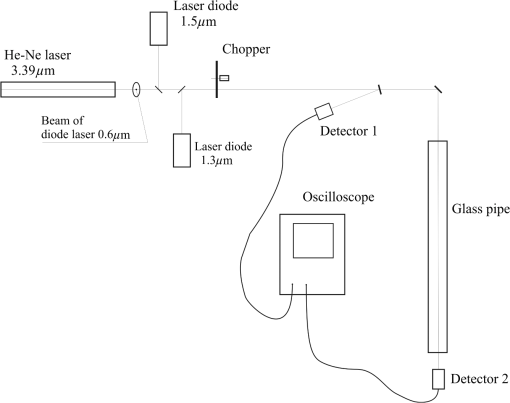
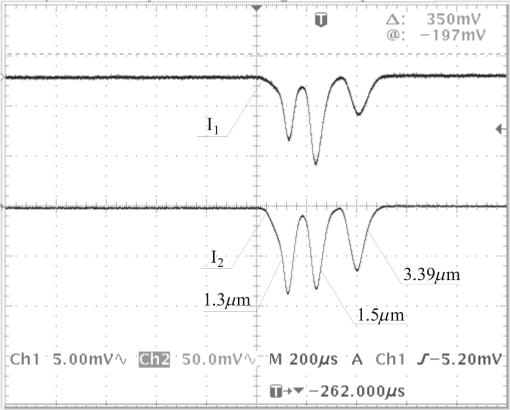
4.Derivation of the Modified Difference Absorption MethodThe commercially available lasers are characterized by relatively high power instability. Moreover, the strong absorption of the 3.39 μm wavelength by a window pane implies the necessity to use detectors with very high sensitivity, which, in the case of lack of window pane, causes saturation of the detectors. These obstacles can be solved by the application of comparative method using two detectors for measuring the optical power before and behind the glass pipe simulating a car and additionally using the reference glass pipe with known optical parameters. The method is schematically presented in Fig. 6. In the absence of any glass pipe, the laser beam is divided by the beam splitter into beam incident on detector 1 and beam incident on detector 2. Because of the instability of the laser, its power can change in time by the index . Thus, after inserting the investigated glass pipe in the laser beam, the intensity can be described as As a result of this, the transmission of the glass pipe can be expressed as Assuming that there is no change of polarization and power distribution in the laser beam, the index can be described as Inserting this expression into Eq. (16), Introducing notations Eq. (17) can be written asInserting the reference glass pipe with known transmission in the laser beam and measuring the intensities and and at the same time introducing the expression , the equation for the transmission can be written as Thus,After inserting this into Eq. (19), the transmission of the investigated glass pipe is Assuming that the reference glass pipe is terminated by glass windows with thickness of and is empty, its transmission can be described as On the other hand, the transmission of the investigated glass pipe filled with alcohol vapor with transmission of terminated by the same windows as in the case of the reference glass pipe, covered with some material with transmission of , can be expressed as where is the distance inside the window that the laser beam has to pass through.After inserting Eqs. (21) and (22) into Eq. (20), For a wavelength , Eq. (23) can be written as If the normal to the window pane with thickness of is not parallel to the laser beam, the distance that the laser light has to pass through inside the window can be described as where is the angle of incidence of the laser beam onto the window pane.Taking into account the calculated refractive indexes from Table 4, the relative difference of for extreme wavelengths (1.3 and 3.39 μm) at can be expressed as In this case, it can be assumed that for Thus Eq. (24) can take the form of where .Assuming that the difference between and is very small (at , ), it can be written that In this case, Eq. (28) for the investigated wavelengths takes the form of After solving the above equations, Inserting the notation Eqs (33), (34), and (35) can be expressed asInserting the calculated absorption coefficients into the above equations, 5.ExperimentThe investigations of the detection of alcohol in the glass pipe simulating a car cabin were carried out in the setup presented in Fig. 7. Four lasers generating at 3.39, 1.5, 1.3, and 0.6 μm wavelengths were used. Their beams were combined into one single beam by glass plates with appropriate thin-layer coatings. The laser generating wavelength at 0.6 μm was used as a pointer to make the adjustment of the system easier. All lasers worked in continuous regime, so the chopper for modulation was used. Small displacement of different laser beams at the surface of the chopper enabled separation of the beams in time domain so as to have been detectable by only two detectors. Two glass pipes with lengths of 140 cm and diameter of 5 cm terminated by the flot glass with thickness of 3.15 mm were used. One of them was the reference glass pipe with clean windows that were perpendicular to the laser beam. The second one was the investigated glass pipe filled with different concentrations of alcohol vapor with windows at angles of 15 deg to the laser beam, covered with graphite dust. Two PbSe detectors were used and the signals were registered by an oscilloscope. In Fig. 8 the picture of the screen of the oscilloscope is presented. The experimental results and calculations are shown in Table 5. Table 5Experimental results and calculations.
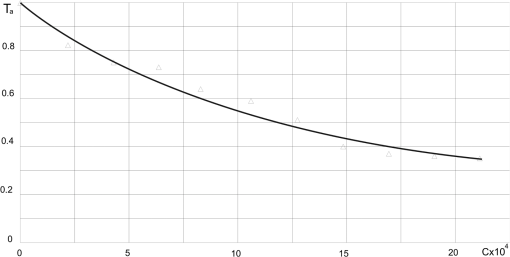
Inserting the calculated in Eq. (42), the transmission of the alcohol can be expressed as To prepare the required concentration of the alcohol vapor inside the investigated glass pipe, the appropriate volume of saturated vapor of alcohol was injected into it with the use of a syringe. On the basis of physical tables, the pressure of saturated alcohol vapor was assumed to be 60 hPa at the temperature of 20°C. Thus the concentration of the alcohol vapor in mole fraction can be described as Changing the units into particle per million (ppm), the concentration can be expressed as Multiplying by the ratio of the volume of the injected alcohol vapor and the volume of the glass pipe (), the concentration of the alcohol vapor in the investigated glass pipe was determined. The results of the investigations for different concentrations of alcohol vapor are shown in Table 6. In Fig. 9 the transmission of alcohol vapor as a function of concentration is presented. Table 6Results of investigations of detection of alcohol vapor at different concentrations.
In the presented experiment the accuracy of measurement of the signals from the detectors appears to be very crucial in order to assure reliable results. Applying the uncertainty theory and assuming that a man having a concentration of alcohol of 0.2‰ in his blood breathes out of it, which corresponds with the alcohol concentration of 50 ppm,17,18 the accuracy of the detection of relative value of the laser radiation should be at least . 6.ConclusionThe results of the experiments prove that the proposed modified difference absorption method of detection of alcohol is reliable. Even if there is an angular tilting of the car windows with respect to the laser beam and car windows are dirty, the proposed solution can work properly. Thus the development of a trustworthy device seems to be feasible. AcknowledgmentsThe work was sponsored by the Polish National Centre for Research and Development, project INNOTECH-K1/IN1/24/153656/NCBR/12. ReferencesR. M. SilversteinG. C. BasslerT. C. Morrill, Spectrometric Identification of Organic Compounds, John Wiley & Sons, New York
(1991). Google Scholar
W. Demtröder, Laser Spectroscopy, Springer-Verlag, Berlin
(2002). Google Scholar
Ø. FarsundG. RustadG. Skogan,
“Standoff detection of biological agents using laser induced fluorescence—a comparison of 294 nm and 355 nm excitation wavelengths,”
Biomed. Opt. Express, 3
(11), 2964
–2975
(2012). http://dx.doi.org/10.1364/BOE.3.002964 BOEICL 2156-7085 Google Scholar
M. Wlodarskiet al.,
“Fluorescence excitation-emission matrices of selected biological materials,”
Proc. SPIE, 6398 639806
(2006). http://dx.doi.org/10.1117/12.687872 PSISDG 0277-786X Google Scholar
G. Feugnetet al.,
“Improved laser-induced fluorescence method for bio-attack early warning detection system,”
Proc. SPIE, 7116 71160C
(2008). http://dx.doi.org/10.1117/12.799143 PSISDG 0277-786X Google Scholar
Z. Mierczyket al.,
“Fluorescence/depolarization LIDAR for mid-range stand-off detection of biological agents,”
Proc. SPIE, 8031–8040 80371J
(2011). http://dx.doi.org/10.1117/12.883866 PSISDG 0277-786X Google Scholar
J. SotorG. SobonK. M. Abramski,
“Er-doped fibre laser mode-locked by mechanically exfoliated graphene saturable absorber,”
Opto-Electron. Rev., 20
(4), 362
–366
(2012). http://dx.doi.org/10.2478/s11772-012-0043-9 OELREM 1230-3402 Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Investigations of optical and generation properties of Yb-Er laser glasses (SELG) designed for 1.5 μm microlasers,”
Proc. SPIE, 6599 65990D
(2007). http://dx.doi.org/10.1117/12.726651 PSISDG 0277-786X Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Optimization of passively repetitively Q-switched three-level lasers,”
J. Quantum Electron., 44
(12), 1152
–1157
(2008). http://dx.doi.org/10.1109/JQE.2008.2003144 IEJQA7 0018-9197 Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Generation investigation of ‘eye-safe’ microchip lasers pumped by 974 nm and 939 nm wavelength,”
Optica Applicata, 38
(4), 657
–668
(2008). OPAPBZ 0078-5466 Google Scholar
J. Mlynczaket al.,
“Comparison of cw laser generation in , : glass microchip lasers with different types of glasses,”
Opto-Electron. Rev., 19
(4), 87
–91
(2011). http://dx.doi.org/10.2478/s11772-011-0048-9 OELREM 1230-3402 Google Scholar
J. Mlynczaket al.,
“Pulse generation at 1.5 μm wavelength in new EAT14 glasses doped with and ions,”
Opto-Electron. Rev., 20
(1), 14
–17
(2012). http://dx.doi.org/10.2478/s11772-012-0003-4 OELREM 1230-3402 Google Scholar
W. Volland, Organic Compound Identification Using Infrared Spectroscopy, Bellevue Community College, Washington
(1999). Google Scholar
S. Bretsznajder, Własności gazów i cieczy, Warsaw
(1962). Google Scholar
Biography Jan Kubicki is a graduate from the Military University of Technology, Warsaw, Poland. In 1980, he received a PhD in the field of molecular lasers. Currently he works at the Institute of Optoelectronics of Military University of Technology. He is an author and co-author of many scientific papers in the field of laser physics, laser spectroscopy and high power laser systems as well as co-author of patents concerning different applications of high power laser pulses and stand-off detection of alcohol.  Jaroslaw Młyńczak received his MSc in 2002 and PhD in 2008 from the Military University of Technology, Warsaw, Poland, where he currently works as a scientist. His research centers on investigation of new active media and new nonlinear absorbers for UV, VIS, IR and “eye-safe” microchip lasers as well as development of microchip lasers for stand-off detection systems. He also participates in research concerning detection of biological agents in the environment as well as biometric identification of people. He is an author and co-author of many scientific and conference papers.  Krzysztof Kopczyński received his MSc in solid-state physics and quantum electronics and PhD in the field of laser physics from the Military University of Technology. He is a director of the Institute of Optoelectronics of Military University of Technology. He is a specialist in the field of physics and technology of solid-state lasers, microchip lasers with selective diode pumping, and laser devices for stand-off detection. He is an author and co-author of a few tens of scientific and conference papers. |