|
|
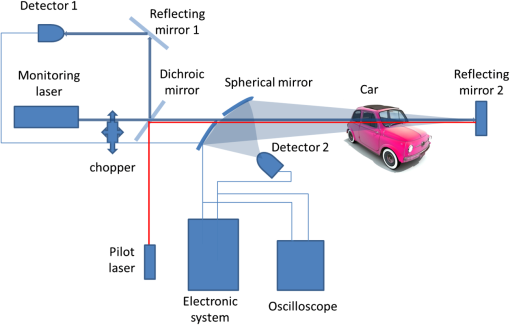
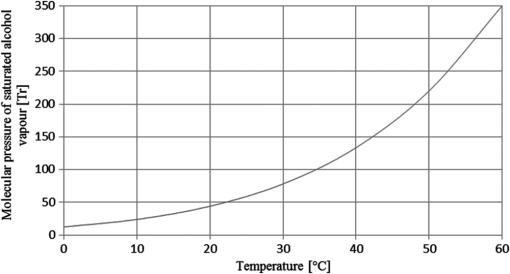
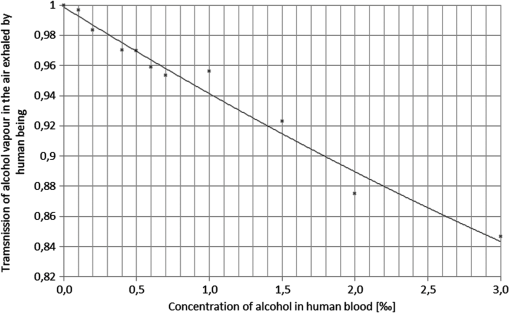
1.IntroductionStand-off detection of different chemical and biological compounds with the use of a monochromatic laser beam at a wavelength fitting into the absorption spectra of these materials is described in many papers.1–6 At the same time, many new developments in lasers that can be used in this application has been achieved in recent years.7–12 Thus, many devices based on this technology were presented in laboratories as well as applied to civil industry, environmental protection, and military. Simultaneously, stand-off detection of alcohol in moving cars draws more and more attention of scientists as well as institutions responsible for safety on the roads,13–16 who are aware of the fact that effective detection of drivers under the influence of alcohol would significantly reduce the number of fatal car accidents. To address this problem, research in this field was taken up at the Institute of Optoelectronics at the Military University of Technology and a special device was designed and built. The configuration of the device was based on the work presented in Ref. 17. The idea of using such device is very simple. It should be deployed by the side of the road to monitor each car passing by. If some vapors of alcohol are detected in a car, a message with a photo of the car including its number plate is sent to a policeman who is waiting by the road several hundred meters further. Then the policeman stops the suspected car driver and checks him using conventional equipment. Sometimes it may occur that the driver is sober while passengers are not or some sort of alcohol was spilt in the car. However, despite this, the idea will surely decrease the number of cars that have to be checked by police and, at the same time, will increase efficacy of stopping drunken drivers. In this paper, we present the results of investigations of the built device in semireal conditions. It means that the real car with special system simulating a driver under the influence of alcohol was deployed on the road while the device was placed in the laboratory. Placing the device in the laboratory made possible to monitor every part of it. 2.Experimental SetupThe investigations of the detection of alcohol in the car cabin were carried out in the setup presented in Fig. 1. The monitoring single-mode laser beam with a wavelength of 3.39 μm and power of 2 mW was directed onto a reflective mirror, which was placed on the other side of the car. The beam was also modulated by a chopper with frequency of 3.6 kHz so as to make the synchronous detection possible. It enabled the detection of signals with amplitudes much lower than the amplitude of noise. Additional laser beam of 0.6 μm wavelength was used as a pilot beam and was aligned with the monitoring beam by a dichroic mirror. The same mirror was also partially reflected for monitoring beam and the partially reflected beam was directed onto the detector 1 to monitor the power of the beam. Checking the monitoring laser beam was necessary because of the fluctuation of its power. To focus the monitoring beam returning from the car onto detector 2, a spherical mirror was used. The spherical mirror had a hole in the middle to let the emitted monitoring beam go through. The signals from the detectors were analyzed by a specially developed electronic system with appropriate signal analysis. To accurately adjust the whole system, an oscilloscope was used. Because the monitoring beam goes through the car two times, the absorption of the beam by the alcohol vapor is increased. Additionally, the displacement of the beam by car windows was also eliminated. On the other hand, this solution caused the increase of absorption of the monitoring beam by the car windows; however, this was overcome by using cooled detectors of high sensitivity. In a real situation, the alcohol vapor comes from the human lung, so in the experiment, this source had to be simulated. It was achieved by evaporation of water solution of alcohol of appropriate concentration and at appropriate temperature. The concentration of alcohol vapor over evaporating solution is determined by Raoult’s law.18 On the basis of this law, the molecular pressure of alcohol vapor over its solution is described by the following equation: where is the mole fraction of alcohol in the solution and is the molecular pressure of saturated alcohol vapor at given temperature.When mixing alcohol of volume with water of volume , mole fraction of alcohol in the solution is given by the following equation: where is the amount of moles of alcohol in solution, is the amount of moles of water in solution, is the molecular weight of alcohol, is the weight of alcohol, is the molecular weight of water, is the weight of water, is the density of alcohol, and is the density of water.Inserting appropriate values from physical arrays,19 mole fraction of alcohol can be written as whereThe molecular pressure of saturated alcohol vapor depends on temperature. In Fig. 2, the molecular pressure of saturated alcohol vapor was presented as a function of temperature.19 When human beings have the concentration of alcohol in their blood equal to 0.2‰, the concentration of alcohol in the exhaled air is equal to , which in turn is equal to 50 ppm ().20 Knowing that normal atmospheric pressure with all vapors is equal to , the molecular pressure of alcohol vapor in such air can be described by the following equation: At the temperature of 25°C, the molecular pressure of saturated alcohol vapor is equal to 59 Tr (Fig. 2). Such pressure will be over the solution of mole fraction equal to . Taking into consideration Eq. (1) it can be written Thus,Inserting Eq. (8) into Eq. (4), Thus,Concluding this derivation, it can be stated that on evaporation of the water solution of alcohol of concentration , the obtained concentration of alcohol vapor in the air is equal to the concentration in the air exhaled by a human being who has a concentration of alcohol in their blood equal to 0.2‰. Thus, one can obtain a specific concentration of alcohol vapor in the air by evaporating the water solution of alcohol of known concentration and at known temperature. 3.ExperimentThe investigated device was deployed in the laboratory close to the open window so as to have the right position to monitor the car that was placed in the path of the monitoring 3.39 μm laser beam. The side windows of the car were closed. On the other side of the car, the reflecting mirror was placed so as to redirect the laser beam in the opposite direction. The configuration of the car and the mirror is presented in Fig. 3. During the experiments, the water solutions of alcohol of concentration between and were evaporated. These correspond to concentration of alcohol in human blood between 0 and 3‰. The measurements were made on the plume of evaporation of alcohol simulating the plume of the air exhaled by the driver. The transmission of this vapor was measured by the device. The results of the investigations are presented in Table 1 as well as in Fig. 4. Table 1Relation between the concentration of alcohol in human blood and the transmission of alcohol vapor in the air exhaled by this human being measured by the device.
4.ConclusionThe results of the investigations presented in this paper show that the developed device works properly. It is able to detect alcohol vapor in a car in which a human being with a concentration of alcohol in blood of at least 0.1‰ is present. Moreover, in real situation, when a person has a concentration of alcohol in blood of 0.1‰, one can expect even higher concentration of alcohol vapor in the exhaled air because the temperature of human’s lung is , which is much higher than in our experiments (25°C). Additionally, even though the transmission of car windows at the wavelength of 3.39 μm is very low and the monitoring beam goes through two windows two times, the power is still high enough to detect the alcohol vapor at such low concentrations. From the practical point of view, there seem to be some countermeasures, such as driving with windows open, solar screens on the side windows, etc., that can be applied by drivers to deceive the system. However, such situations are very easily detected by the system, which sends this information to the policeman indicating that the car should be checked. In case of driving with air-conditioning or fans on, the results of measurements will surely be distorted, but it depends on the speed of moving air, which usually is very low and, thus, its impact on the measurements is slight. All the possible countermeasures are very important and have to be investigated, which will be done during the next stages of the ongoing project. As far as the commercialization of the device is concerned, the next step would be to make it more compact, robust, and customer friendly, which can be achieved with high probability. AcknowledgmentsThis work was sponsored by the Polish National Centre for Research and Development, project INNOTECH-K1/IN1/24/153656/NCBR/12. ReferencesR. M. Silverstein, Spectrometric Identification of Organic Compounds, John Wiley & Sons, New York
(1991). Google Scholar
W. Demtröder, Laser Spectroscopy, Springer-Verlag, Berlin
(2002). Google Scholar
Ø. FarsundG. RustadG. Skogan,
“Standoff detection of biological agents using laser induced fluorescence—a comparison of 294 nm and 355 nm excitation wavelengths,”
Biomed. Opt. Express, 3
(11), 2964
–2975
(2012). http://dx.doi.org/10.1364/BOE.3.002964 BOEICL 2156-7085 Google Scholar
M. Wlodarskiet al.,
“Fluorescence excitation-emission matrices of selected biological materials,”
Proc. SPIE, 6398 639806
(2006). http://dx.doi.org/10.1117/12.687872 PSISDG 0277-786X Google Scholar
G. Feugnetet al.,
“Improved laser-induced fluorescence method for bio-attack early warning detection system,”
Proc. SPIE, 7116 71160C
(2008). http://dx.doi.org/10.1117/12.799143 PSISDG 0277-786X Google Scholar
Z. Mierczyket al.,
“Fluorescence/depolarization LIDAR for mid-range stand-off detection of biological agents,”
Proc. SPIE, 8037 80371J
(2011). http://dx.doi.org/10.1117/12.883866 PSISDG 0277-786X Google Scholar
J. SotorG. SobonK. M. Abramski,
“Er-doped fibre laser mode-locked by mechanically exfoliated graphene saturable absorber,”
Opto-Electron. Rev., 20
(4), 362
–366
(2012). http://dx.doi.org/10.2478/s11772-012-0043-9 OELREM 1230-3402 Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Investigations of optical and generation properties of Yb-Er laser glasses (SELG) designed for 1.5 μm microlasers,”
Proc. SPIE, 6599 65990D
(2007). http://dx.doi.org/10.1117/12.726651 PSISDG 0277-786X Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Optimization of passively repetitively Q-switched three-level lasers,”
J. Quantum Electron., 44
(12), 1152
–1157
(2008). http://dx.doi.org/10.1109/JQE.2008.2003144 IEJQA7 0018-9197 Google Scholar
J. MlynczakK. KopczynskiZ. Mierczyk,
“Generation investigation of ‘eye-safe’ microchip lasers pumped by 974 nm and 939 nm wavelength,”
Optica Applicata, 38
(4), 657
–668
(2008). OPAPBZ 0078-5466 Google Scholar
J. Mlynczaket al.,
“Comparison of cw laser generation in Er3þ,Yb3þ: glass microchip lasers with different types of glasses,”
Opto-Electron. Rev., 19
(4), 87
–91
(2011). http://dx.doi.org/10.2478/s11772-011-0048-9 OELREM 1230-3402 Google Scholar
J. Mlynczaket al.,
“Pulse generation at 1.5 μm wavelength in new EAT14 glasses doped with Er3þ and Yb3þ ions,”
Opto-Electron. Rev., 20
(1), 14
–17
(2012). http://dx.doi.org/10.2478/s11772-012-0003-4 OELREM 1230-3402 Google Scholar
J. KubickiJ. MlynczakK. Kopczynski,
“Application of modified difference absorption method to stand-off detection of alcohol in simulated car cabins,”
J. Appl. Remote Sens., 7
(1), 073529
(2013). http://dx.doi.org/10.1117/1.JRS.7.073529 1931-3195 Google Scholar
S. Bretsznajder, Własności gazów i cieczy, Wydawnictwo Naukowo-Techniczne, Warsaw
(1962). Google Scholar
W. Wojtowicz, Tablice Matematyczno-Fizyczne, Państwowe Zakłady Wydawnictw Szkolnych, Warsaw
(1972). Google Scholar
G. AwsiukiewiczT. Mikulski, Badanie stanu trzeźwości w służbie przy użyciu urządzeń kontrolno-pomiarowych, Zakład Interwencji Policyjnych Szkoły Policji, Słupsk
(2013). Google Scholar
BiographyJarosław Młyńczak received his MSc in 2002 and PhD in 2008 from the Military University of Technology, Warsaw, Poland, where he currently works as a scientist. His research centers on investigation of new active media and new nonlinear absorbers for UV, VIS, IR, and “eye-safe” microchip lasers as well as development of microchip lasers for stand-off detection systems. He also participates in research concerning detection of biological agents in the environment as well as biometric identification of people. He is an author and coauthor of many scientific and conference papers. Jan Kubicki is a graduate from the Military University of Technology, Warsaw, Poland. In 1980, he received a PhD in the field of molecular lasers. Currently, he works at the Institute of Optoelectronics of Military University of Technology in the field of laser physics and high-power laser systems. He is an author and coauthor of many scientific papers in the field of laser physics, laser spectroscopy, and high-power laser systems. Krzysztof Kopczyński received his MSc in solid-state physics and quantum electronics and a PhD in the field of laser physics from the Military University of Technology. He is a director of the Institute of Optoelectronics of Military University of Technology. He is a specialist in the field of solid-state lasers, microchip lasers, and laser devices for stand-off detection. He is an author and coauthor of a few tens of scientific and conference papers. |